Product Launches 2019
Cordis
MYNX CONTROL™ Vascular Closure Device: SECURE EXTRAVASCULAR CLOSURE
MYNX CONTROL™ VCD provides active extravascular sealing and resorbability properties with a next-generation delivery system to maximize predictability, safety, and ease of use in sealing 5-7F femoral arterial access sites.
Featuring:
- A next-generation deployment system designed for predictability and ease of use with a 2-button design to simplify procedural steps
- A sheath catch that is compatible with the procedural sheath
- A tension indicator that provides visual confirmation of device position for proper sealant deployment
- Available in 5F as well as 6/7F sizes
MYNX™ VCD has been clinically proven to reduce surgical complications, expedite recovery, shorten hospital stays, and increase patient comfort (1-5).
Visit Cordis at booth #52 to learn more!
1 Pruski MJ Jr et al. MynxGrip for closure of antegrade puncture after peripheral interventions with same-day discharge. Vasc Endovasc Surg. 2017 Feb;51(2):67-71.
2 Baker NC et al. Active versus passive anchoring vascular closure devices following percutaneous coronary intervention: a safety and efficacy comparative analysis. J Interv Cardiol. 2016 Feb; 29(1): 108-112.
3 Hutchings D et al. Success, safety, and efficacy of the Mynx femoral closure device in a real-world cohort: single-center experience. J Invasive Cardiol. 2016 Mar;28(3):104-108.
4 Noor S et al. Successful reduction of surgeries secondary to arterial access site complications: a retrospective review at a single center with an extravascular closure device. Vasc Endovascular Surg. 2010 Jul;44(5):345-349.
5 Fargen KM et al. A prospective randomized single-blind trial of patient comfort following vessel closure: extravascular synthetic sealant closure provides less pain than a self-tightening suture
Philips Image Guided Therapy
See clearly. Treat optimally.
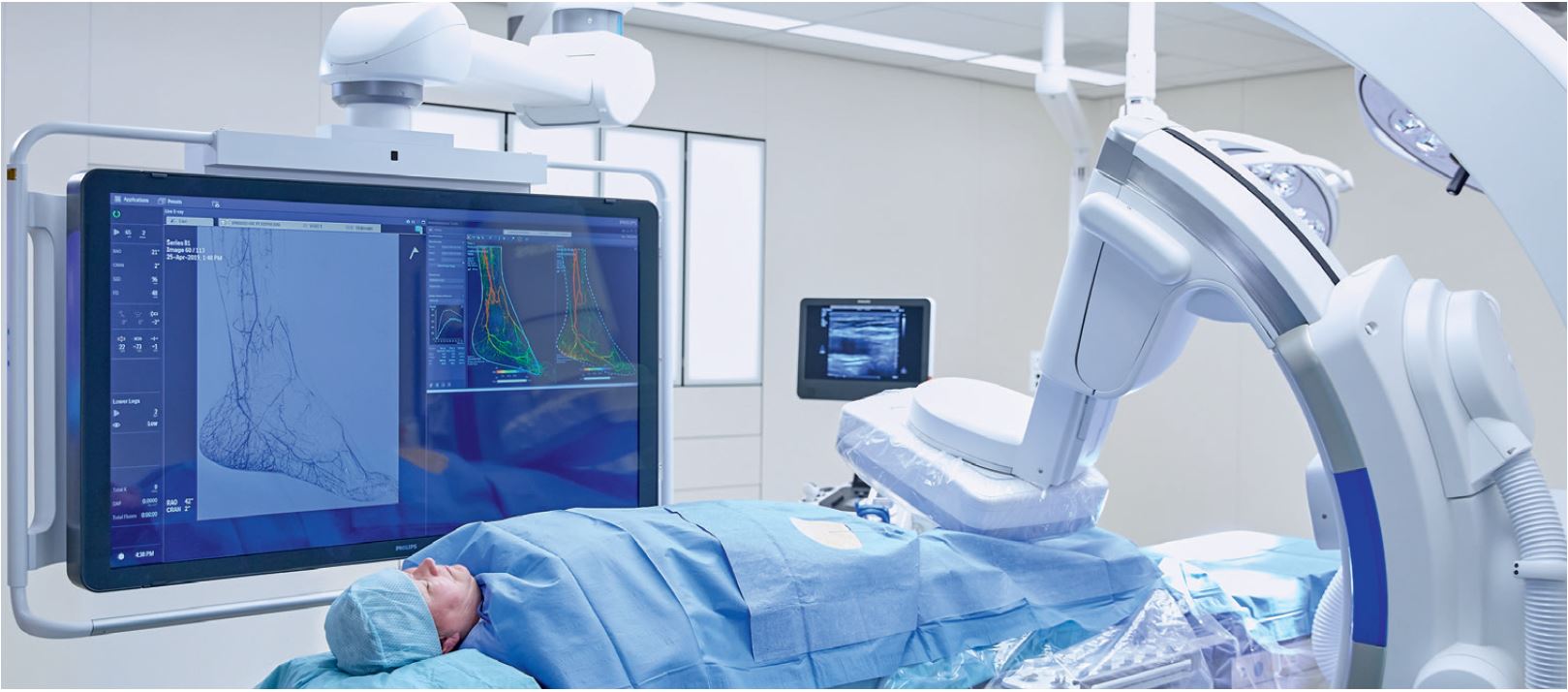
Philips Azurion with FlexArm –
the advanced suite that works around you.
Philips Azurion 7 C20 with FlexArm is a revolutionary new approach to image-guided therapy, giving you the freedom to improve and grow your minimally invasive care.
This new ceiling-mounted system provides unlimited imaging flexibility for interventional radiology procedures, and exceptional positioning freedom for medical teams. All of this in a compact set-up, providing a highly cost-effective environment ready for the procedures of the future. By working around you, Philips Azurion with FlexArm helps optimize your suite performance, so you can deliver superior care.
- Stop by the Philips booth to discover how Azurion with FlexArm empowers you to deliver better clinical outcomes
in interventional radiology with:
SmartPerfusion – perfusion imaging technology that provides you with an objective understanding of the impact of your patients’ treatment, helping you determine the outcome of CLI procedures. - Philips Devices – offering the world’s first dedicated BTK IVUS platform to complement crossing solutions, atherectomy, drug-coated balloons and existing intravascular imaging (IVUS), for peripheral vascular, aorta, and deep venous procedures.
Join us at booth #3 and attend our Lunch Symposium on September 7th in Auditorium 2 (13:00 to 14:00) to discover more about FlexArm, SmartPerfusion, IVUS and many more.
www.philips.com/flexarm
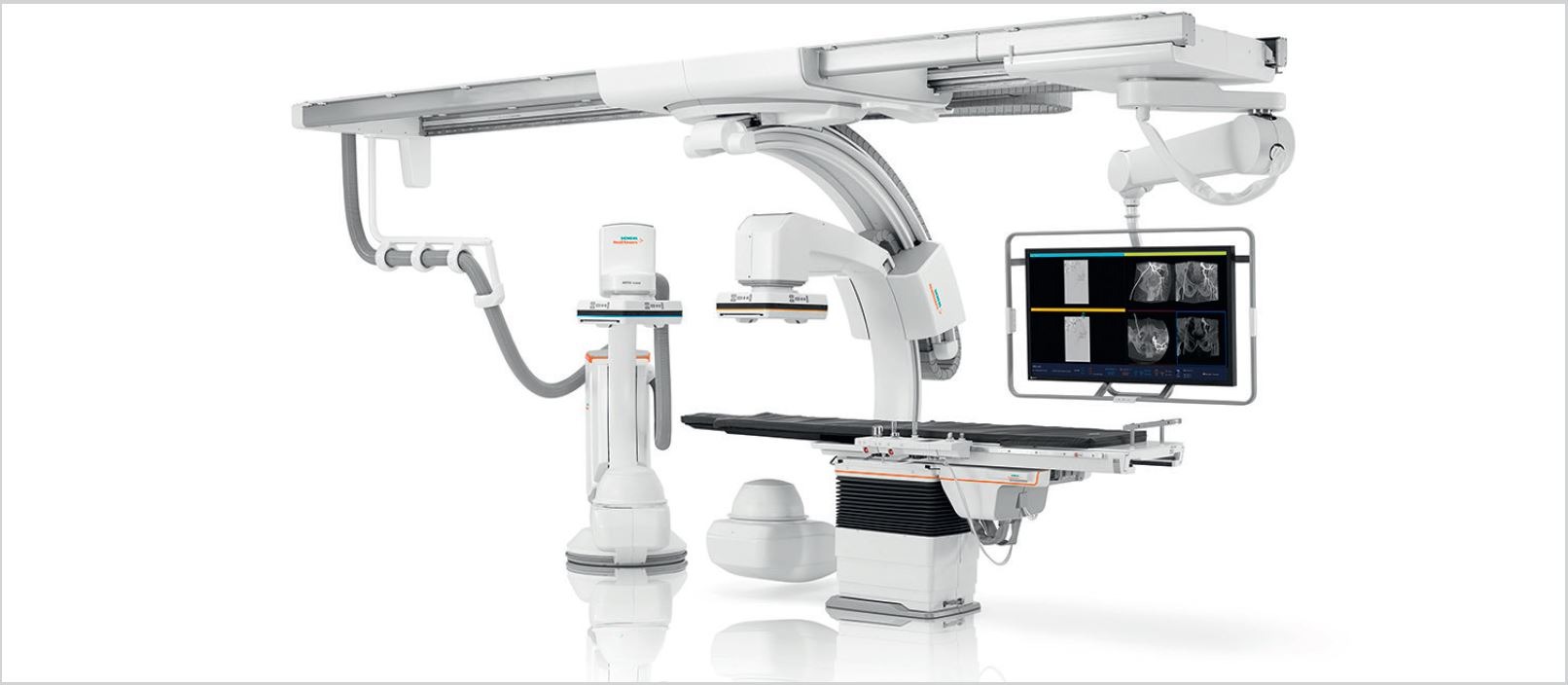
Siemens Healthineers
Transforming care delivery in image-guided therapy
Minimally invasive interventions hold a vast potential for growth and innovation: the ARTIS icono family was designed to help you realize that potential.
Multi-disciplinary usage between different clinical specialties and procedural intelligence for efficient workflows will allow to transform care delivery and expand precision medicine. Optimize your clinical operations with Case Flows, a sequence of system settings matching the diagnostic steps and treatment path.
For an extraordinary visibility of details regardless of patient size and C-arm angulation, ARTIS icono features OPTIQ, a novel CNRbased image chain. Driven by intelligent, self-adjusting algorithms, it results in constant image quality independent of angulation. ARTIS icono allows you to fully focus on the procedure.
ARTIS icono. An icon of innovation.
siemens-healthineers.com/artis-icono
The ARTIS icono system and its features are not commercially available in all countries.
Future availability cannot be guaranteed.
Terumo Interventional Systems
MEDSPHERE
MEDSPHERE is a complete Radiofrequency Ablation System that allows the physician to ablate soft tissue in organs such as Liver, Kidney, Lung, Thyroid, as well as bone (osteoid osteoma). The system can be used with or without cooling system.
The versatility of MEDPSHERE can expand RFA ablation options, supporting the physician in choosing the best solution to approach each lesion and organ.
In fact, MEDSPHERE offers a wide range of treatment options:
- Two ablation modalities: Power Mode and Temperature mode
- Two electrode shapes: retractable Umbrella electrodes and Straight Cooled electrodes
- Various Gauges: 15G and 17G for Umbrella electrodes; 16G, 17G, 18G and 19G for straight cooled electrodes.
- Various sizes: umbrella diameters 2cm, 3cm, 4cm, active tips from 5mm, 10mm, 15mm, 20mm, 30mm.
- Various lengths: 7cm, 10cm, 15cm, 20cm, 25cm
All electrodes have a detachable cable, for an easy positioning and management during CT operations.
An intellegent protection mechanism stops RF delivery in case of malfunctioning.
QuiremScout®
QuiremScout® is the first and only CE-marked SIRT work-up product that utilizes the same technology as the therapeutic microspheres which aims to optimize patient selection and advance treatment planning
QuiremScout® has been shown to be more accurate than the commonly used surrogate 99mTc-MAA at predicting lung shunting1 and intrahepatic distribution2
It has also been proven to be clinically safe in a population of 82 patients3