CIRSE Insider: Prof. Helmberger, can you briefly explain the aim of CIRT and CIRT-FR? What makes these studies significant?
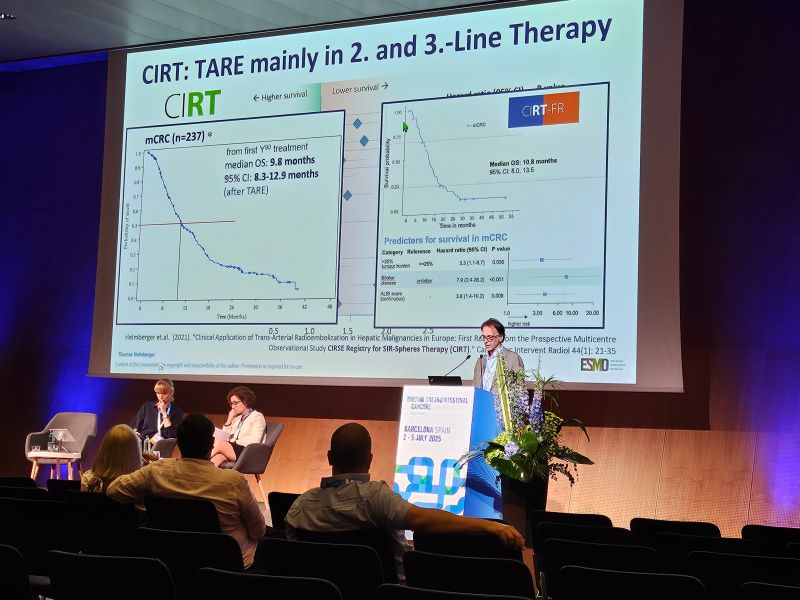
Helmberger: CIRT’s goal was to capture outcomes in routine clinical practice for both primary and secondary liver cancers, thereby complementing clinical trial data with robust “real-world” evidence. CIRT-FR is the France-specific extension of this project, mandated by the French health authorities as an offshoot of CIRT. CIRT-FR aimed to gather comprehensive data on patients treated with Y-90 resin microspheres in France, specifically to assess treatment safety, effectiveness, and quality of life under national reimbursement criteria. In fact, the French authorities made reimbursement for certain TARE patients conditional on collecting this real-world evidence.
These studies are significant because they represent one of the largest real-world evidence efforts in interventional oncology for liver cancer. Additionally, because of the large scale of CIRT, we’ve been able to identify new prognostic indicators – for example, an analysis of the European CIRT data highlighted the AST-to-Platelet Ratio Index (APRI) as a potential predictor of survival after TARE. Insights like these, along with the confirmed quality-of-life data, help doctors refine patient selection and aftercare.
CIRSE Insider: CIRT and CIRT-FR collected real-world data from medical centres on a multinational and national scale, respectively. Prof. Bilbao, what is the value of collecting real-world data in IR research?
Bilbao: Registries with real-world data allow a better understanding of functionalities, applications, and treatments in a daily practice setting. They allow us to obtain robust safety and efficacy data, enable data collection on less common tumours, and give relevant information concerning long-term patient outcomes.